How to find finalized consent forms?
August 20, 2019
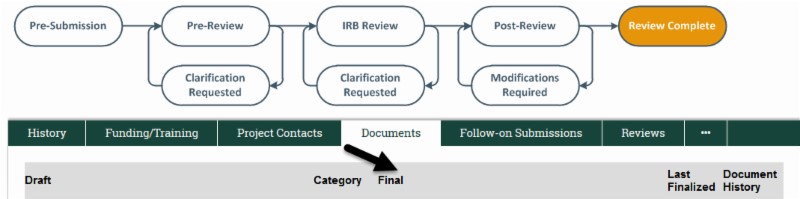
If applicable, use the IRB approved finalized consent (with IRB approval language indicating an effective date) to enroll subjects. To find the finalized version of the consent, navigate to the study’s workspace and click on the “Documents” tab. From there, the finalized version will be available under the “Final” column. Remember, if you are obtaining a signed consent you must provide a copy of the consent to subjects. A copy of the signed consent must be provided to the subject if you are following ICH-GCP.